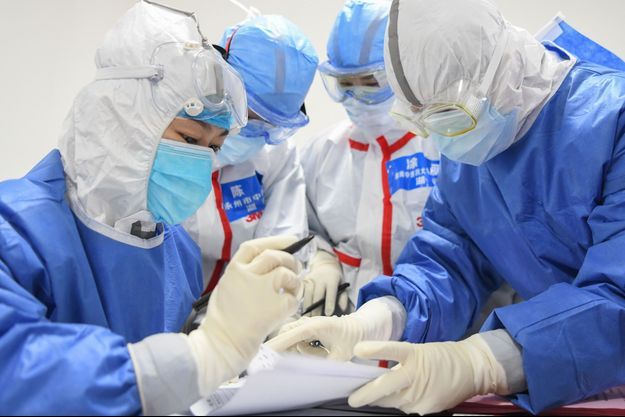
In recent weeks, scientists have sounded the alarm about new variants of the coronavirus that carry a handful of tiny mutations, some of which seem to make vaccines less effective.
But it is not just these small genetic changes that are raising concerns. The novel coronavirus has a propensity to mix large chunks of its genome when it makes copies of itself. Unlike small mutations, which are like typos in the sequence, a phenomenon called recombination resembles a major copy-and-paste error in which the second half of a sentence is completely overwritten with a slightly different version.
A flurry of new studies suggests that recombination may allow the virus to shapeshift in dangerous ways. But in the long term, this biological machinery may offer a silver lining, helping researchers find drugs to stop the virus in its tracks.
Some scientists are studying recombination machinery not only to fend off the next pandemic, but to help fight this one.
For example, in his recent study on the recombination of three coronaviruses, Dr. Denison of Vanderbilt found that blocking an enzyme known as nsp14-ExoN in a mouse coronavirus caused recombination events to plummet. This suggested that the enzyme is vital to coronaviruses’ ability to mix-and-match their RNA as they replicate.
Now, Dr. Denison and Sandra Weller, a virologist at the University of Connecticut School of Medicine, are investigating whether this insight could treat people with Covid.
Other scientists see potential in this approach, not only to make drugs like remdesivir work better, but to prevent the virus from fixing any of its replication mistakes.
“I think it’s a good idea,” Dr. Goldstein said, “because you would push the virus into what’s known as ‘error catastrophe’ — basically that it would mutate so much that it’s lethal for the virus.”
Oxford releases new data on vaccine efficacy against UK strain, Johnson & Johnson seeks FDA approval, and the US Senate passes a key resolution for coronavirus aid. Here’s what you should know:
First the bad news: There’s some evidence that B.1.1.7, the variant first discovered in the UK, could be more deadly than the original coronavirus strain, in addition to being more transmissible. Now the good news: New data released Friday indicate that the Oxford-AstraZeneca vaccine protects against both the original virus and this mutation. This research hasn’t yet been peer-reviewed, but it’s promising. Though a handful of new, allegedly more contagious variants have made it stateside in recent weeks, the CDC warned last month that B.1.1.7 could be the predominant variant in the US by March.
In response, drugmakers are rushing to retool shots to boost protection against variants. And on Thursday, the FDA said that it’s putting together new standards for adapting drugs, tests, and vaccines specifically to fight against more resilient mutations. These will likely be released in the next two to three weeks. The plan builds on years of experience with the flu virus, which changes quickly and constantly. The National Institutes of Health is also working with at least two drugmakers to start human trials of variant-targeting vaccines next month.
On Thursday, Johnson & Johnson applied for emergency use authorization from the FDA for its Covid-19 vaccine. If approved, the vaccine will likely go into use in late February or early March. Supplies are expected to be limited at first, but the shot will still be a welcome addition. Unlike the two vaccines currently in use, it requires only one dose and can be stored in a regular refrigerator. Still, the fact that Johnson & Johnson’s vaccine has a slightly lower efficacy rate than those already in use means that officials may soon face difficult questions about who gets which shot.
Indeed, equity issues have already proven central to vaccination, especially as distribution ramps up under the Biden administration. People with ample free time and internet access have had an easier time getting vaccinated, which disadvantages many of the groups that have been disproportionately affected by the pandemic, including Black, Latinx, and indigenous people. On Tuesday, the White House announced expansions of the vaccination program that will, among other things, prioritize vaccination efforts in minority communities.
After hours of voting, the Senate approved a budget resolution at 5:30 am on Friday that brings legislators one step closer to passing Biden’s $1.9 trillion coronavirus relief bill with a simple majority, rendering Republican support unnecessary. Biden’s plan includes $1,400 per-person direct payments for most households, a weekly $400 unemployment insurance supplement through September, expanded paid leave, and more. Earlier this week, the president met with a group of GOP senators to discuss an alternate $618 billion relief plan they had drafted in an apparent attempt to work across the aisle, but he later reaffirmed that he wants Democratic legislators to “go big” on pandemic aid.
These developments in Washington come as economic recovery is stalling nationwide. While the US economy added 49,000 jobs last month, the pace of job gains remains sluggish. Economists say things will likely accelerate as more people get vaccinated and pandemic restrictions on businesses ease up.
For people who are pregnant, the rollout of COVID-19 vaccines is prompting agonizing questions about whether it’s safer to get the vaccine or risk infection. Despite emerging evidence that the vaccines are generally safe and effective, there is virtually no data as to whether that’s true for those who are expecting, even though they are at higher risk of complications from the disease.
The world’s regulatory bodies have at times issued contradictory advice about pregnancy and COVID-19 vaccines. The Centers for Disease Control and Prevention (CDC) has said that the vaccines should be available to pregnant people but ultimately leaves the decision up to expectant parents and their doctors. The World Health Organization (WHO) recommends against it unless the pregnant person is at high risk
So how does someone make an evidence-based decision about whether it’s safe to get the vaccine in the absence of any safety data? “It all turns on the features of your life,” says Ruth Faden, founder of the Johns Hopkins Berman Institute of Bioethics in Maryland. Each person must balance what is known about the vaccine with what is known about their own risk of getting infected.
Although experts suggest talking through these decisions with a medical provider, here's a look at the facts available, what’s still being sorted out, and why there’s reason to be optimistic.
The Moderna and Pfizer-BioNTech vaccines for COVID-19 pose a new challenge. Until now, the messenger RNA platform they use had not been licensed for human use. As such, the only pregnancy-related data available are from preclinical studies in laboratory animals and a handful of clinical trial participants who later discovered they were pregnant. (Here's the latest on COVID-19 vaccines.)
But we do know a fair amount about how the mRNA technology works. Instead of using inactivated or live virus, these vaccines contain snippets of genetic code encased in lipids, or fat globules, that protect the code from degrading. Once injected, the mRNA instructs cells to produce the SARS-CoV-2 spike protein, which triggers the body’s immune response.
Theoretically, all of this is promising because, like past vaccines, it does not involve a live virus. “Everything that is understood to be biologically the case about mRNA vaccines is incredibly reassuring,” Faden says. “It shouldn’t have any impact on pregnancy or pregnancy outcomes.”
Anthony Fauci, White House chief medical adviser, has also said that the data “so far has no red flags” for pregnant people.
Still, scientists have raised questions about how the mRNA vaccines will work in reality. The biggest concern is whether mRNA can cross the placenta and generate the spike protein in the fetus. It wouldn’t necessarily be harmful if it did—and would not cause birth defects—but the worry is that the fetus could experience side effects including pain, swelling, and fever. Swamy says the animal studies showed no signs of physical side effects, but that is yet to be tested in humans.
Side effects in the mother may also be an issue. Christina Chambers, a perinatal epidemiologist at the University of California, San Diego, is conducting a study of COVID-19 vaccinated pregnant women. She notes that it can be harmful to the baby when a pregnant woman runs a high fever. “If that is a side effect, you’d want to pay attention to that and talk to your provider about taking something to reduce the fever,” she says.
There are clinical trials in the pipeline to investigate the effects of the vaccines in pregnant women. Faden wishes these trials had started as soon as the vaccines received FDA approval, but she points out that the process is still moving more rapidly than it has in the past.
“We used to feel like one or two lonely drums out there, beating our drums in this vast silence,” she says. “Now we’ve got like a whole percussion section calling for more data and the inclusion of pregnant women in the rollout of the vaccine. And that’s a really good thing.”